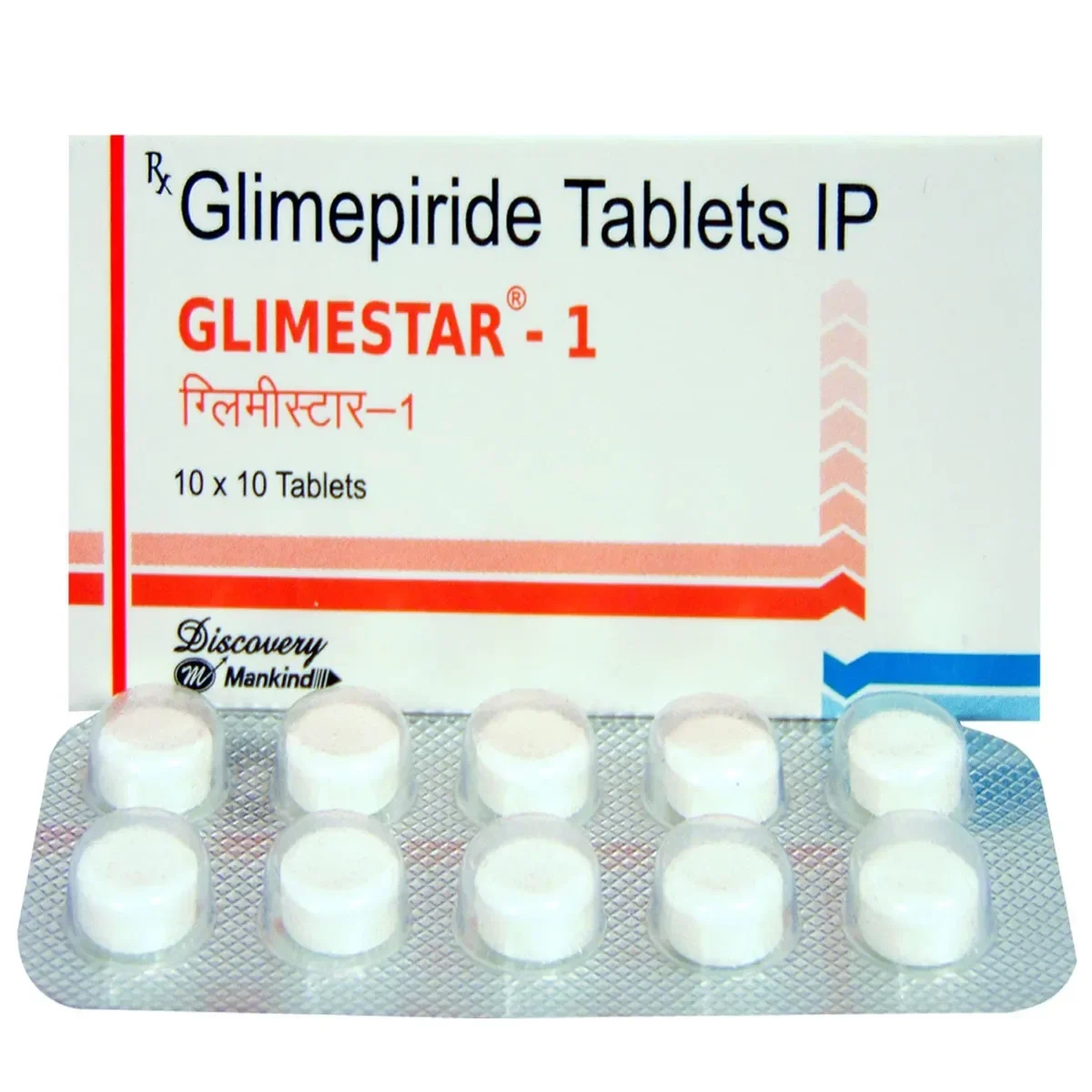
Glimestar 1 Tablet – Effective Type 2 Diabetes Management
Glimestar 1 Tablet is a sulfonylurea-class antidiabetic medication that helps adults manage type 2 diabetes mellitus by controlling blood sugar levels. Consistent use reduces the risk of diabetes-related complications, including kidney damage, nerve disorders, and vision problems.
Key Features
-
Category: Sulfonylurea antidiabetic
-
Indication: Type 2 diabetes mellitus
-
Form: Tablet
-
Administration: Oral, preferably before or with the first meal of the day
Benefits
Glimestar 1 Tablet promotes insulin release from the pancreas, lowering blood sugar levels and supporting:
-
Improved blood glucose management
-
Reduced risk of complications such as heart, kidney, and nerve damage
-
Long-term health benefits when combined with diet, exercise, and weight control
Common Side Effects
Most side effects are mild and resolve on their own:
-
Low blood sugar (hypoglycemia)
-
Headache
-
Nausea
-
Dizziness
-
Weakness
Recognize low blood sugar symptoms such as sweating, shaking, and dizziness. Carry fast-acting glucose and avoid alcohol to prevent hypoglycemia.
How to Use
Take Glimestar 1 Tablet exactly as prescribed by your doctor. Swallow the tablet whole with food at the same time daily. If a dose is missed, skip it and continue with your regular schedule; do not double the dose.
Safety Information
-
Pregnancy & Breastfeeding: Consult your doctor; may not be suitable.
-
Kidney & Liver: Use with caution; dose adjustments may be required.
-
Driving: Blood sugar fluctuations may affect alertness; avoid driving if symptoms occur.
-
Alcohol: Unsafe while taking this medication.
Mechanism of Action
Glimestar 1 Tablet works by stimulating insulin secretion from the pancreas, helping to maintain stable blood glucose levels and supporting overall diabetes management.
Product Variants & Keywords
Glimestar 1 Tablet, Glimestar Tablet, Sulfonylurea Antidiabetic Tablet, Type 2 Diabetes Medicine, Blood Sugar Control Tablet, Diabetes Management Tablet
Vendor Information
- Address:
- No ratings found yet!





















Reviews
There are no reviews yet.